Custom Vaccine Development

|

|

|
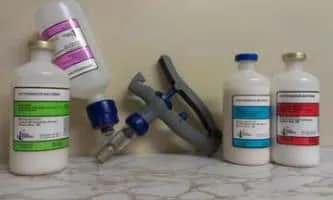
| CNVS 20/20 Foot Vac | CNVS Pneu Vac 3 | CNVS Pneu Vac 3+M |
|
CNVS 20/20 Foot Vac Fusobacterium necrophorum causes “Footrot” septic arthritis and liver abscesses. This product contains two highly virulent strains taken from actual foot rot cases in our practice territory. Foot rot is a common contagious condition that can plague cattle all year around on pasture, and in feedlots. It can cripple the animal for life as well as significantly reduce gain and performance. Moraxella bovoculi is a newly emerged cause of infections involving the eye commonly called “Pinkeye”. This is our first custom vaccine available in our area from pinkeye caused by M. bovoculi. Because of the prevalence of M. bovoculi many commercially prepared vaccines have not been effective in preventing pinkeye. Our clinical experience indicates that if current pinkeye vaccines are not effective for you, you most likely have M. bovoculi as a significant agent in your herd. We continue to search for new strains of bacteria from Footrot and Pinkeye cases so we can improve and maintain this custom autogenous product. This product is also combined with the adjuvant Trigen®. We are aware of no other product commercially available with the customer acclaimed history of combining this all in one vaccine. CNVS 20/20 Foot Vac can be given subcutaneous as a one-dose product but in high-risk operations or in the face of a challenge, we do recommend two doses as we do with all killed vaccines. CNVS 20/20 Foot Vac goes through the same USDA safety, purity, and sterility requirements as do commercially available “Name Brand” vaccines. Because of our ability to culture bacteria specific to your area we can custom design this and other products in attempt to provide better and more specific immunity to pathogens in your area. Our client history of custom vaccine satisfaction suggest you too will be satisfied. It has been used exclusively by our practice clientele since 2006 with no adverse reactions. ************************************ CNVS SCOUR VAC *********************************** CNVS Pneu Vac 3+Myco CNVS Pneu -Vac 3 is a custom made multi-stain Manhemia (Pasteurella) haemolytica, Pasteurella multocida, Histophilus somni, Mycoplasma bovis vaccine. These 4 respiratory anitgens are the cause of predominately all bacterial pneumonia problems in cattle. CNVS Pneu Vac 3+Myco is unique that it contains customer satisfied protection against pneumonia caused by all four, utilizing cell lines that are known to be a problem in our area. Mycoplasma pneumonia is on the rise and there is no consistently effective treatment available. Two strains of Mycoplasma are inluded as we feel prevention of Mycoplasma pneumonia is a must. We continue to search for new strains of respiratory bacteria so we can improve and maintain this custom autogenous product. This product is combined with the adjuvant Trigen®. It is also made with a duel fermentation process that enhances leukotoxin protection which is necessary for proper Mannhemia and Pasteurella specific protection. We are aware of no other product commercially available with the customer acclaimed history of doing this all in one vaccine. CNVS Pneu Vac 3+Myco can be given subcutanously as a one-dose product but in high-risk operations or in the face of a challenge, we recommend two doses as we do with all killed respiratory vaccines CNVS Pneu Vac 3+Myco has been used by our satisfied practice clientele since 2001 with no known adverse reactions. ************************************ CNVS Autogenous 20/20 Foot Vac* **Comparisons of CNVS Autogenous 20/20 Foot Vac to other manufactured pinkeye and foot rot vaccines**
*Autogenous vaccines are federally approved and are an alternative to traditional commercial vaccines. They are specifically formulated and have special use regulations. For more information contact CNVS @ 888-269-8387 or your local veterinarian. **No claim of efficacy or superiority is made or implied. This table is only for information and comparison of bacterial types and/or strains contained in each product as advertised. * Multiple X’s represent the number of bacterial types and/or strains present. ************************************ CNVS Autogenous Scour Vac* **Comparisons of CNVS Autogenous Scour Vaccine to other manufactured scour vaccines**
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
*AUTOGENOUS VACCINES ARE FEDERALLY APPROVED AND ARE AN ALTERNATIVE TO TRADITIONAL COMMERCIAL VACCINES. THEY ARE SPECIFICALLY FORMULATED AND HAVE SPECIAL USE REGULATIONS. FOR MORE INFORMATION CONTACT CNVS @ 888-269-8387 OR YOUR LOCAL VETERINARIAN. **NO CLAIM OF EFFICACY OR SUPERIORITY IS MADE OR IMPLIED. THIS TABLE IS ONLY FOR INFORMATION AND COMPARISON OF BACTERIAL TYPES AND/OR STRAINS CONTAINED IN EACH PRODUCT AS ADVERTISED. * MULTIPLE X’S REPRESENT THE NUMBER OF BACTERIAL TYPES AND/OR STRAINS PRESENT. |
